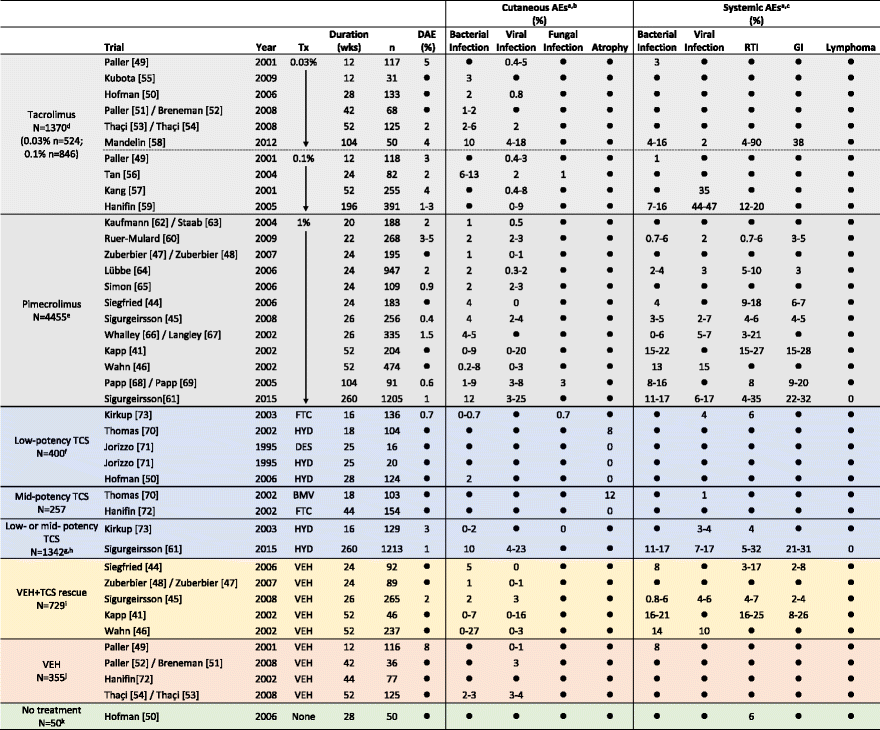
- If trial duration was <1 year and reported in months, the number of weeks was calculated as follows: duration in weeks = 4 X duration in months. If trial duration was ≥1 year, the number of weeks was calculated as follows: duration in weeks = number of years X 52
- Only incidences of adverse events (AEs) that were specifically reported to be “0” are indicated as such; AEs that were not a specified study outcome and/or were not reported are indicated as “●”
- Incidences of discontinuations due to adverse events (DAEs) and AEs >1 % are rounded to the nearest whole number; incidences are presented as a range when multiple AEs within the same category were reported
-
a AEs that may have been reported in the studies but are not shown include: application site reactions (burning, pruritus, etc.), potentially atopic or allergic events, or events with unknown/unclear origin (asthma, conjunctivitis, coughing, fever/pyrexia, joint pain, nasal congestion, nasopharyngitis, pharyngitis, rhinitis/coryza, rhinorrhea)
-
b Cutaneous AE categories
-
Bacterial infection = abscess, bacterial infection, boil, cellulitis, eczema Infected/infection, erysipelas, folliculitis, furuncle, impetigo, infection, pustules, staphylococcal Infection, streptococcal infection, or stye
-
Viral infection = chicken pox, eczema herpeticum, flat warts, herpes simplex, herpes virus infection, herpes zoster, Kaposi’s varicelliform eruption, molluscum, skin papilloma, varicella, viral rash Candida, fungal infection, ringworm
-
Atrophy = antecubital fossae atrophy, atrophy, hypertrichosis, popliteal fossae atrophy, telangiectasia
-
c Systemic AE categories
-
Bacterial infection = bacterial pneumonia, bronchitis, ear infection, external otitis, infection, laryngitis, sinusitis, Staphylococcus infection, Streptococcus pharyngitis, tonsillitis
-
Viral infection = flu-like symptoms, influenza, influenza-like Illness, viral encephalitis
-
Respiratory tract infection (RTI) = otitis media, respiratory tract infection, upper respiratory tract infection, viral respiratory tract infection
-
Gastrointestinal infection (GI) = diarrhea, gastroenteritis, viral gastroenteritis, vomiting
-
d Incidences of some viral infections were reported only for the combined tacrolimus group in the Paller study
-
e Results for the 2 pimecrolimus treatment groups are combined in the Ruer-Mulard and Whalley/Langley studies
-
f TCS treatment was received In the Hofman study as an active control for tacrolimus
-
g Patients received either hydrocortisone 1 % (low-potency TCS) or hydrocortisone butyrate 0.1 % (mid-potency TCS)
-
h TCS treatment was received in the Sigurgeirsson study as an active control for pimecrolimus
-
i TCS was permitted to treat flares not controlled by study medication
-
j Tacrolimus was permitted in the Paller/Breneman and Thaçi/Thaçi studies for flares not controlled by study medication
-
k These subjects did not have AD
-
AE indicates adverse event, BID twice daily, BMV betamethasone valerate, DAE discontinuations due to AE, DES desonide, FTC, fluticasone propionate, GI gastrointestinal, HB hydrocortisone butyrate, HYD hydrocortisone, mo months, PM pimecrolimus, pts patients, QD once daily, RTI respiratory tract infection, TC tacrolimus, TCS topical corticosteroids, Tx treatment, URTI upper respiratory tract infection, VEH vehicle, wk, week(s), yr, year(s)

